this post was submitted on 02 Oct 2024
1703 points (95.6% liked)
Microblog Memes
9290 readers
3677 users here now
A place to share screenshots of Microblog posts, whether from Mastodon, tumblr, ~~Twitter~~ X, KBin, Threads or elsewhere.
Created as an evolution of White People Twitter and other tweet-capture subreddits.
Rules:
- Please put at least one word relevant to the post in the post title.
- Be nice.
- No advertising, brand promotion or guerilla marketing.
- Posters are encouraged to link to the toot or tweet etc in the description of posts.
Related communities:
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
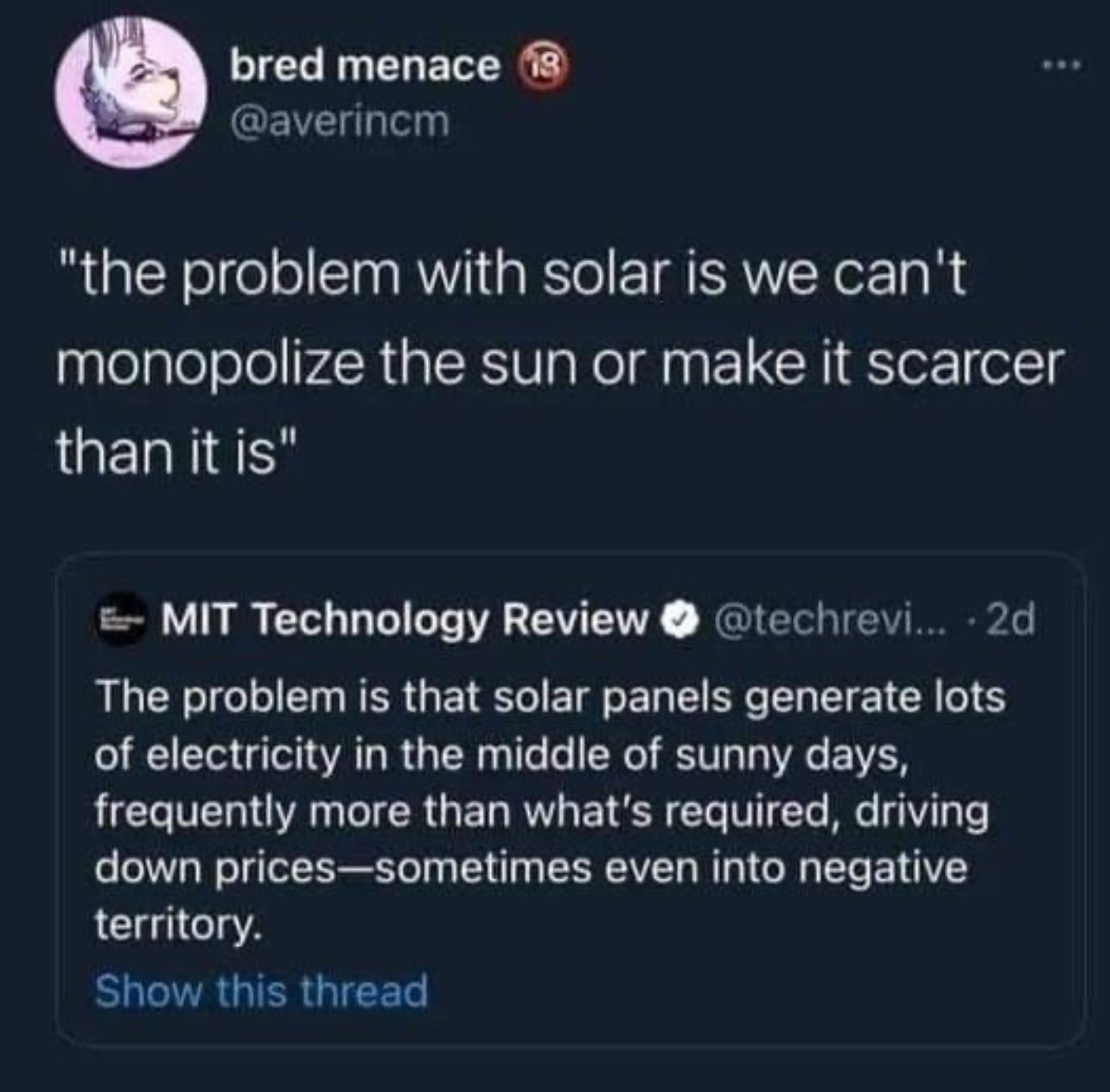
Or use it to generate hydrogen for simpler, cheaper, more reliable, sustainable hydrogen powered cars.
We don't even have enough lithium to replace the average country's existing cars, let alone all of them, or literally anything else that requires lithium.
Not sure where our good buddy @Hypx@fedia.io went, but let me assure you. As of right now, 100% of available hydrogen stocks are fossil fuels derived.
Hydrogen vehicles being green is a fantasy pedaled by fossil fuel companies to not have to move away from natural gas. While it is possible to generate hydrogen through electrolysis, functionally, none actually is. It's far far cheaper to do so from natural gas, and probably always will be.
Promoting hydrogen as a "solution" is basically promoting fossil fuels green washing.
And I'm not sure where you are getting you information on lithium, but it's probably the best short and medium term option. Beyond that, gravity storage (pump water up hills, and maybe some kind of hydrogen system that doesn't require transporting the stuff where it can be made and stored in place when solar or wind energy is abundant.
Most battery chemistries are moving away from rare earth metals like lithium. Solid state batteries are the next step, and they use things like sodium cloride, I.E salt, as their base.
What that article describes sounds like an awesome development. Too bulky for vehicles at the moment, but possibly excellent for grid storage.
Isn't one the issues with hydrogen motors that they are a bit explodey? Genuine question, haven't looked into it in a long time.
Pure hydrogen doesn't explode. It's only if you mix it with oxygen. The Hindenberg glowed red not blue
Good thing there's no oxygen around then. Petrol doesn't burn without oxygen either, but it's still dangerous. Additionally typical fuel cell hydrogen cars, store the hydrogen in tanks up to 10,000 psi, which is where the explosion part happens.
Agreed. Petrol cars are also explodey. As are EVs. In fact most energy dense objects are explodey.
The issue with the 10000 psi tanks are the size and weight. Not the explodeyness.
Another huge expensive problem is transporting it is not easy. At room at atmospheric pressure and temperature, it takes up like 2-3 grams per gallon of space, making it super inefficient to transport.
You could pressurize it, but that makes it insanely flammable and a risk of it leaks. You could also cryo-freeze it, but that is also very expensive to transport, it require a lot of energy to freeze it, maintain it during long transports, and to unfreeze it at it's destination.
Building a hydrogen delivery infrastructure is probably the best way to overcome this, but that would also take years and billions.
I'm no expert on the field, but I'd imagine a lot of energy departments would rather do that cost and effort towards building new green energy plants that can deliver power to grids rather than only help cars. Car-wise, most things are transitioning to hybrid or electric anyways, so they also benefit from a green power plant.
The only way I've seen hydrogen make sense is where it's made and stored on site for later grid level generation. Transporting it makes very little sense for all the reasons you mentioned. Salt concerns and ammonia have both been discussed as potential storage options. But you wouldn't move it around. Store it in a fixed location and generate the electricity on site. If you don't have to move it, hydrogen might make some sense.
https://www.mdpi.com/1996-1073/13/12/3062
Hydrogen is a pain to deal with. It requires excessively thick walled containers to store etc.
A better solution is to do what plants do. Pin it to a carbon atom. Synthetic hydrocarbons would also be a lot easier to integrate into existing supply chains.
Where's the carbon going to come from? If it's anywhere but the CO2 in the atmosphere (or at least sequestered on its way to the atmosphere), your energy solution isn't carbon neutral anymore. And if it is from the atmosphere, then there are efficiency challenges there at concentrating CO2 to be useful for synthetic processes.
Most syngas today comes from biological and fossil feedstocks, so it's not really a solution to atmospheric CO2 concentrations.
There are a lot more ways to store energy other than lithium and hydrogen.
Pumped storage, vanadium redox battery, sodium battery, ... I'd even say they are most suited for grid-level energy storage.
I have doubts that hydrogen will ever work in any industry, but it definitely won't work for cars. The storage and distribution challenges are never going to make it cost competitive with just regular lithium batteries on a marginal per-joule basis. Even if the energy itself is free, the other stuff will still be more expensive than just charging car batteries off the existing grid.